Abstract
Background: Sickle cell disease (SCD) is an inherited, lifelong disorder characterized by sickle hemoglobin (HbS) polymerization, resulting in red blood cell (RBC) sickling and destruction, hemolysis, anemia, vaso-occlusion, and end-organ damage. Therapies for SCD have historically been limited, and treatment for RBC sickling remains an unmet need. While voxelotor is a first-in-class HbS polymerization inhibitor, GBT021601 is a next-generation agent that stabilizes hemoglobin (Hb) in the oxygenated state, inhibits polymerization, and has potential to be a best-in-class therapy. Studies are underway to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics (PD) of GBT021601. Innovative PD biomarker data helps streamline drug development and maximize treatment benefit by providing early indicators of treatment effect.
Objective: To describe how PD biomarker data provides insight into the effectiveness and proof-of-concept data for GBT021601.
Methods: Two studies, which are the first to administer oral GBT021601 loading and maintenance dose regimens at 50 mg, 100 mg, 150 mg daily in adults with SCD, are in progress: 1) a phase 1, single-arm, intrapatient, single-dose and multiple-ascending-dose (MAD) study (NCT04983264), and 2) a phase 2/3, randomized, multicenter study (NCT05431088).
In the phase 1 study, PD markers such as p20 and p50 (partial pressures of O2 at which Hb is 20% and 50% saturated with O2, respectively) were determined by measuring oxygen equilibrium curves (OECs) after 2 dose escalations. OECs were generated by hemoximetry, which relates the extent of Hb-O2 saturation to the partial pressure of O2 and measures the binding affinity of O2 to Hb (Metcalf B, 2017; doi: 10.1021/acsmedchemlett.6b00491). Additionally, oxygen gradient ektacytometry, which measures RBC deformability under oxygenated and deoxygenated conditions, was implemented to evaluate the impact of GBT021601 on RBC deformability. The cerebral hemodynamic effects of GBT021601 are being evaluated in a subset of patients from the phase 1 study using diffuse correlation spectroscopy (DCS) and frequency domain near infrared spectroscopy (FDNIRS), which are well-validated, noninvasive optical techniques, to assess the relationship between cerebral blood flow, oxygen extraction fraction, cerebral metabolic rate for oxygen, and change in Hb levels. Regional microvascular oxygen saturation, blood flow, and oxygen metabolism, which are measured by DCS and FDNIRS, may serve as novel biomarkers for treatment efficacy.
In the phase 2/3 study, PD biomarkers similar to those used in the phase 1 study will be assessed.
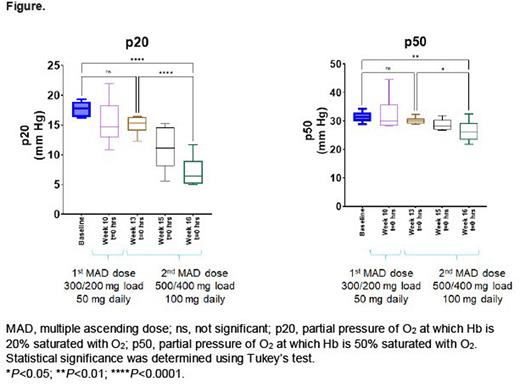
Results: In the phase 1 study, hemoximetry box plots (Figure) show p20 and p50 values of blood collected from patients with SCD (n=6) during dosing with GBT021601. The p20 and p50 values for baseline (week 8) to week 10 and weeks 13 to 16 represent the 50 mg and 100 mg GBT021601 dosing periods, respectively. Both the p20 and p50 values decreased from baseline, indicating Hb modification of blood by GBT021601 and a dose-dependent increase in Hb-O2 affinity. These changes in p20 and p50 values during treatment with GBT021601 correspond to left shifts of the OECs relative to baseline (no treatment). The change in p20 per dose is larger than that with p50 and represents a more sensitive measure of Hb modification and indicator of increased Hb-O2 affinity. In the phase 2/3 study, the ektacytometry evaluation between doses suggests a dose response between 50 and 100 mg.
Preliminary data in participants administered 150 mg daily dosing on safety, tolerability, PD markers, and cerebral hemodynamics, as measured by DCS and NIRS, are forthcoming.
Conclusion: Loading and maintenance dose regimens of 50 and 100 mg GBT021601 achieved a targeted steady-state drug concentration that corresponded to improvements in hemoximetry and ektacytometry in adults with SCD. Results indicate that OEC measurements allow sensitive monitoring of the degree of increase in Hb-O2 affinity and represent Hb modification by GBT021601 during treatment.
Use of novel biomarkers provides insights on the effect of GBT021601. Data from these studies will provide a better understanding of the impact of this next-generation treatment on RBC health and oxygen delivery in patients with SCD.
Funding: Global Blood Therapeutics and the National Science Foundation Graduate Research Fellowship Program under Grant No. 1937971 (ROB).
Disclosures
Idowu:Pfizer: Research Funding; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees; Forma: Research Funding; Ironwood: Research Funding. Nguyen:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Buckley:Global Blood Therapeutics: Research Funding. Dufu:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Lisbon:Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal